Researchers connect molecular function to high blood pressure, diseases
By changing one small portion of a stimulus that influences part of one molecule's function, engineers and researchers at Washington University in St. Louis have opened the door for more insight into how the molecule is associated with high blood pressure, autism and movement disorders
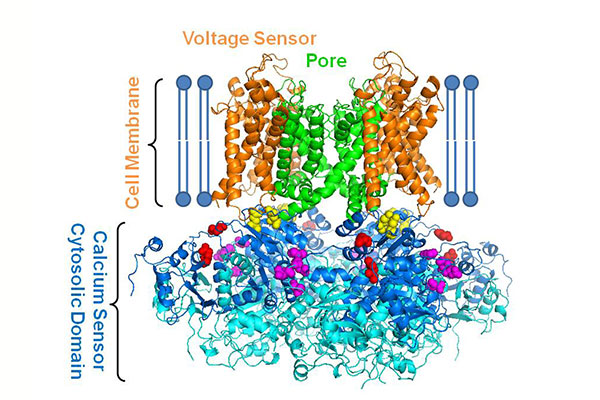
The finding, published online Feb. 14 in the Journal of General Physiology, lays the foundation for further insight into mechanisms behind the connection of the molecule with these and other diseases, such as epilepsy and circadian rhythm disorders.
Cells have ion channels, which are pathways that regulate current through the cell membrane and open in response to physical signals, such as voltage, or chemical signals, such as calcium, potassium or sodium. But these channels typically allow one type of ion to pass through, for example, the BK (big potassium) channel, only allows potassium to pass through.
Jianmin Cui, professor of biomedical engineering in the School of Engineering & Applied Science, and collaborators in three labs at WashU are studying the BK channel, which has been found to be important in regulating neuronal function and blood pressure.
"This channel is interesting in two ways: one, because it is physiologically very important, and two, because the mechanism that makes it work is very interesting and quite unique and provides a way to study how the physiological signal is used to open ion channels," Cui says.
Unlike most ion channels, the gate that opens and closes the BK channel is activated by a change in voltage and by calcium ions, but scientists do not understand how they work together to open the channel. To answer this question, Cui's co-author and collaborator, Lawrence Salkoff, professor of neuroscience and of genetics at the School of Medicine, found that when he removed a portion of the protein for calcium stimulus to open the channel, voltage still worked to open the pore.
"We have been interested in how the two stimuli open the channel separately and how they interact with each other and affect each other when they open the channel together," Cui says. "When we cut off the intracellular part of the channel that is responsible for calcium stimulus, we got confirmation that voltage can still open the channel independently from the calcium stimulus. What is not known is how the two interact with each other and affect each other to open the channel, so we've been looking at how the voltage mechanism opens the channel alone and what the calcium influences."
Salkoff's truncated BK channel allows the investigators to examine how the intracellular domain, which serves as the calcium sensor, to influence voltage stimulation of channel opening.
"It turns out that the removal of the intracellular domain weakens the connection between the voltage sensor and the pore, making the channel less responsive to the changes of voltage sensor conformation," Cui said. "It indicates that calcium may influence the voltage stimulus by regulating the connection between the voltage sensor and the pore."
Cui says the finding creates even more questions about this function in this important ion channel, the activity of which also has implications in smooth muscles including the lungs and uterus.
Funding for this research was provided by the National Institutes of Health (R01 HL70393, R01 NS092570, R01 GM114694) and the National Science Foundation of China (31271143).




