Understanding low-temperature plasma has technological, human benefits
Thimsen earns NSF CAREER Award
Most of us know about solids, liquids and gasses. But there is a fourth state of matter — plasma, a partially ionized gas. As a gas-like substance that can conduct electricity, plasma is useful for a range of technologically-valuable applications from lasers to manufacturing computer chips.
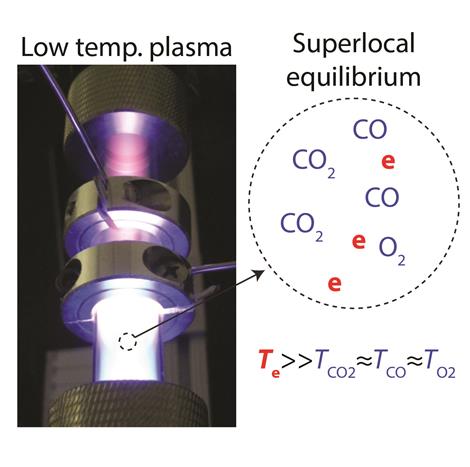
At equilibrium, plasma is the stable state of matter at very high temperatures of thousands of degrees, for example on the surface of the sun. In the laboratory, it is possible to produce low temperature plasma by selectively exciting electrons to very high temperatures while the atoms and molecules in the system remain near room temperature. Low temperature plasma is a highly nonequilibrium state of matter.
Elijah Thimsen, of the McKelvey School of Engineering at Washington University in St. Louis, will study how chemical reactions occurring in low-temperature plasma move toward a superlocal equilibrium state with a five-year, $500,000 CAREER Award from the National Science Foundation. The awards support junior faculty who model the role of teacher-scholar through outstanding research, excellent education and the integration of education and research within the context of the mission of their organization. One-third of current McKelvey Engineering faculty have received the award.
Thimsen, assistant professor of energy, environmental & chemical engineering, will explore the idea of a superlocal equilibrium state, in which a system is constrained to have different temperatures at the same location in space, depending on the species; and entropy is maximized. That idea is a reasonable way to describe low temperature plasmas from the perspective of thermodynamics, since the gas molecules are near room temperature but the electrons are very hot due to the electricity flowing into the system, he said.
Thermodynamics dictates that normally a chemical reaction will proceed towards the local equilibrium state. For example, carbon monoxide will react with oxygen to form carbon dioxide and the reaction will stop since carbon dioxide is the dominant species at equilibrium.
"A common analogy is if you are driving somewhere, thermodynamics tells you where your destination is," he explained. "There are many paths to that destination, and you don't know whether or not you'll get there or what path you'll take, but thermodynamics will tell you where a chemical reaction is headed."
Typically, the local equilibrium state is assumed to be the final destination of the chemical reaction, which is defined by the temperature, the total pressure and the relative amounts of different elements in the system, Thimsen said.
"What we're proposing in this project is that in a system governed by superlocal equilibrium, such as a low-temperature plasma, the destination of the chemical reaction is determined by, in addition to those three variables, we will add two more: the electron temperature and the electron concentration," he said. "The proposal is that the destination of the chemical reaction, which is the superlocal equilibrium state, is then determined by those five variables."
Thimsen and his team are using carbon dioxide (CO2) as a first example to illustrate the idea.
"Experimentally, if we feed CO2 into the reactor at a background temperature and pressure where at equilibrium you expect pure CO2, the plasma causes it to split spontaneously into carbon monoxide and oxygen," he said. "The amount of carbon monoxide and oxygen increases with time in the reactor and saturates at a constant value consistent with the system approaching steady state. Moreover, if we feed CO plus oxygen into the reactor in the same relative amounts of the elements, then at long times the composition is the same as when pure CO2 was fed into the reactor, provided we keep the five variables constant. Thus, the system appears to be approaching a superlocal equilibrium state, independent of the initial condition, provided the five state variables have the same values."
Advances in understanding low-temperature plasma could improve plasma activation of advanced carbon fiber composites used to make aircraft and automobiles more fuel efficient. In addition, they could benefit greenhouse gas emissions, plasma agriculture and plasma medicine, protecting the environment and human health.
In addition to the research in his lab, Thimsen will be working with a chemistry teacher, Elisabeth Knierim, from Belleville West High School to improve understanding of thermodynamic equilibrium by students in the Advanced Placement chemistry classes.
"The goal is to reinforce the idea of equilibrium as a terminal state that the system proceeds toward," he said. "And furthermore, emphasize that the equilibrium state depends on the local conditions."