Protein’s role in immune system after heart attack to get a closer look
Matthew Bersi plans to investigate the role of a protein in potential immunotherapeutic treatment strategies for inflammatory cardiovascular disorders
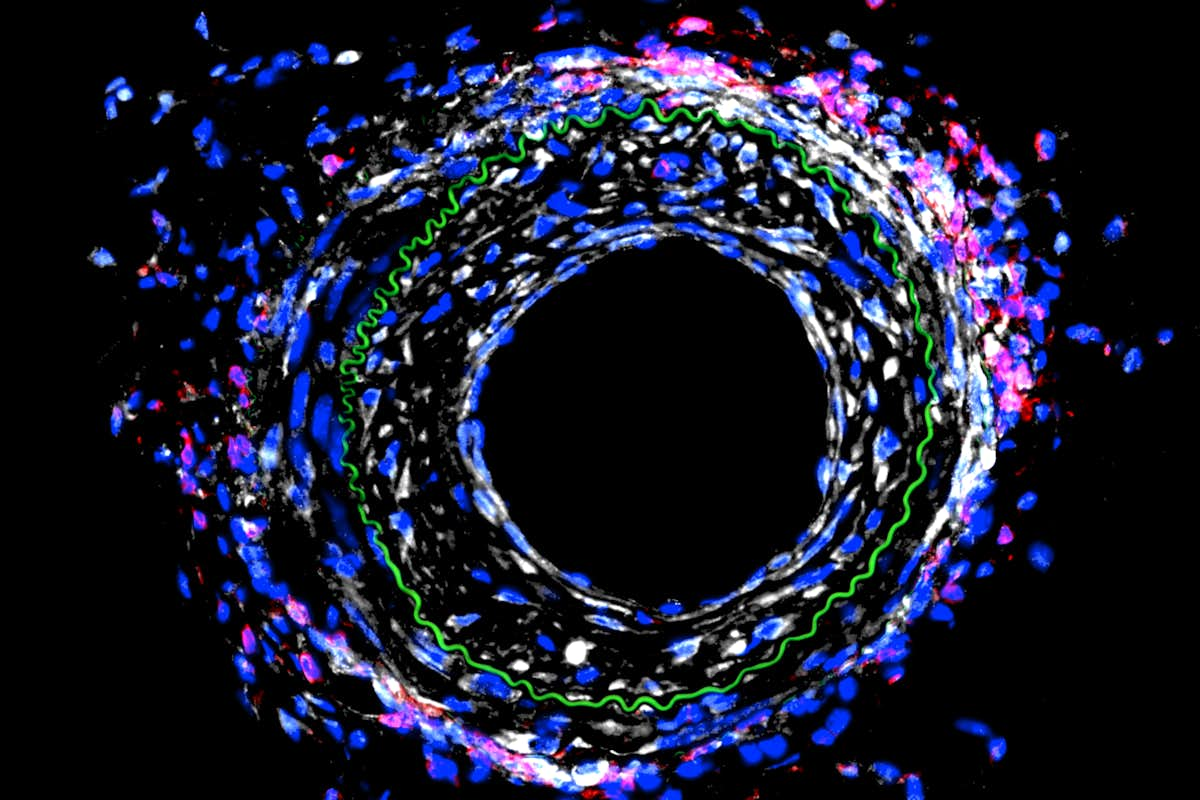
Heart attacks are caused by one or more blocked blood vessels, and physicians can restore blood flow in the heart with a stent or by bypassing the blockages with new vessels. A mechanical engineer at Washington University in St. Louis plans to take a closer look at how those blood vessels remodel in response to blocking a particular protein and the effect of that action on the immune system.
Matthew Bersi, assistant professor of mechanical engineering & materials science in the McKelvey School of Engineering, received a three-year, $750,000 grant from the National Institutes of Health (NIH) to study the role of the cadherin-11 protein in the mechanical injury of blood vessels after a heart attack and how cells respond to promote disease. The grant is a continuation of NIH-funded mechanobiology research he began as a postdoctoral researcher at Vanderbilt University.
Previously, Bersi found in a mouse model that blocking activity of the cadherin-11 protein after a heart attack had a beneficial effect in the heart — the heart was not as damaged as it would have been with the protein, but had a detrimental effect on the remodeling of blood vessels.
“In the early work, we found that by modulating the activity of this protein, we’re affecting the immune system, and that change in the immune system is causing the disease to be worse in the vasculature,” Bersi said. “Now, we’re investigating the mechanisms behind that to identify new directions in the treatment of this pathology. Our hypothesis is that we would alter the immune activity contributing to the disease and reduce the vessel remodeling.”
Cadherin-11 acts as a receptor on the outside of cells that binds to and signals to other cells. This activity may lead to injuries to cells or to fibrosis, where the tissue gets stiff and hard. A heart with fibrotic tissue doesn’t function normally and can lead to heart failure.
“With a mechanical injury in a vessel wall, cells may be responding in a way that is changing how the immune system responds to that injury,” Bersi said. “We’re looking at the relationship between the mechanobiology of the tissue and immunobiology, or how the immune system responds to the mechanical responses of the cells in the tissue.”
Bersi plans to continue the research in a mouse model and to develop a computational model as well, both in an effort to identify potential immunotherapeutic treatment strategies for cardiovascular disorders fueled by inflammation.




