Wagenseil to look at elastin’s role in aortic stenosis development
Jessica Wagenseil is taking a closer look at the role of the protein elastin in this condition to provide new information that may reveal the cause and help treat the disease in the future.
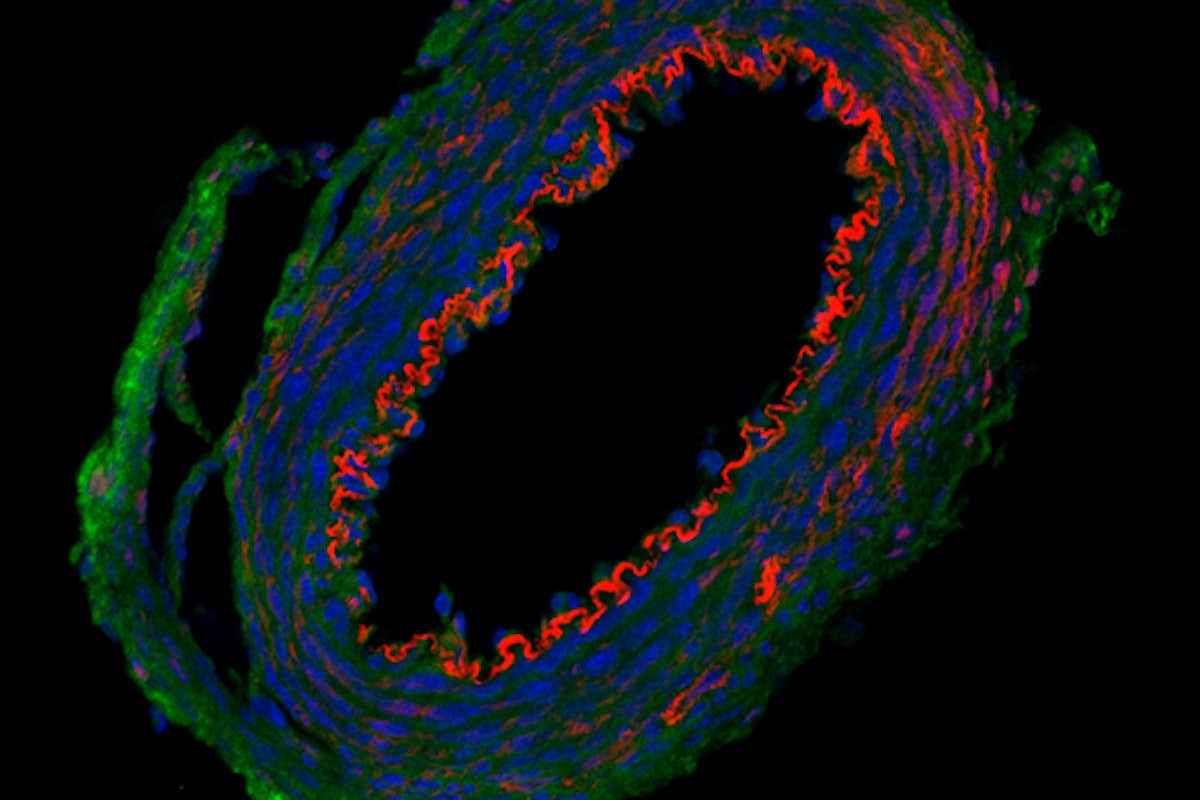
Supravalvular aortic stenosis is a congenital defect that leads to the narrowing of the aorta, the large blood vessel that carries blood from the heart to the rest of the body. A mechanical engineer at Washington University in St. Louis is taking a closer look at the role of the protein elastin in this condition to provide new information that may reveal the cause and help treat the disease in the future.
Jessica Wagenseil, associate professor of mechanical engineering & materials science in the McKelvey School of Engineering, will study elastin, a protein that provides elasticity in connective tissue, in normal aortic development and how reduced elastin levels contribute to stenosis with a one-year, $393,750 grant from the National Institutes of Health. The new research builds on work published in Circulation Research in 2019 in which Wagenseil, in collaboration with Chien-Jung Lin, MD, PhD, a cardiology fellow, and Robert Mecham, the Alumni Endowed Professor of Cell Biology & Physiology, both at Washington University School of Medicine, and others on her team developed a new mouse model that showed how smooth muscle cells and endothelial cells contribute elastin to the arterial wall and how local elastin defects may contribute to cardiovascular disease.
“The key is that we can control what cells are making the elastin and when,” she said of the paper’s results. “That tool allows us to investigate different questions of how much elastin we need and which cells are making it.”
Elastin gets deposited in the aortic wall by smooth muscle cells during a critical window of embryonic development. If there is a mutation in the elastin gene, it can lead to elastin insufficiency and aortic stenosis. There are no ways to increase elastin, so the only treatment for stenosis is surgical repair or replacement to allow blood to flow through the artery freely to restore proper heart function. Children born with the mutation often face multiple surgeries throughout their lives.
Using the mouse model, Wagenseil and her team will investigate the minimum elastin levels needed to prevent stenosis and the mechanisms that lead to reduced elastin during development, which she said is critical to understand.
“Elastin has a 70-year half-life,” Wagenseil said. “Once we make it, it’s supposed to last for a lifetime. If there is not enough, or it’s not in the right places, we want to figure out how to fix that.”
Lin C-J, Staiculescu M, Hawes J, Cocciolone A, Hunkins B, Roth R, Lin C-Y, Mecham R, Wagenseil J. Heterogeneous Cellular Contributions to Elastic Laminae Formation in Arterial Wall Development. Circulation Research, Nov. 8, 2019. DOI: 10.1161/CIRCRESAHA.119.315348.




