Bai to study dynamic heterogeneities in lithium-ion battery electrodes
This NSF CAREER Award will help Bai’s lab further its understanding of the significance and evolution of spatiotemporal heterogeneities in batteries, complementing earlier work
Lithium-ion batteries (LIBs) have revolutionized the way people live, by enabling transformative electronic devices, portable power tools and electric vehicles. They are popular choices of energy storage technologies, wherever high energy density, high power density and system simplicity are required. However, elusive safety accidents, especially life-threatening fires and explosions, have become a major and urgent concern. Failures of LIBs and other high-energy batteries always originate from microscopic heterogeneities, which however are difficult to be monitored, analyzed, and predicted by existing electroanalytical methods.
Peng Bai, assistant professor of energy, environmental and chemical engineering has been preparing to better understand the dynamic heterogeneities in the electrodes of lithium-ion and other high-energy batteries, and now received a five-year, $503,025 CAREER Award from the National Science Foundation (NSF). The NSF CAREER awards support junior faculty who model the role of teacher-scholar through outstanding research, excellence in education and the integration of education and research within the context of the mission of their organization. One-third of current McKelvey Engineering faculty have received the award.
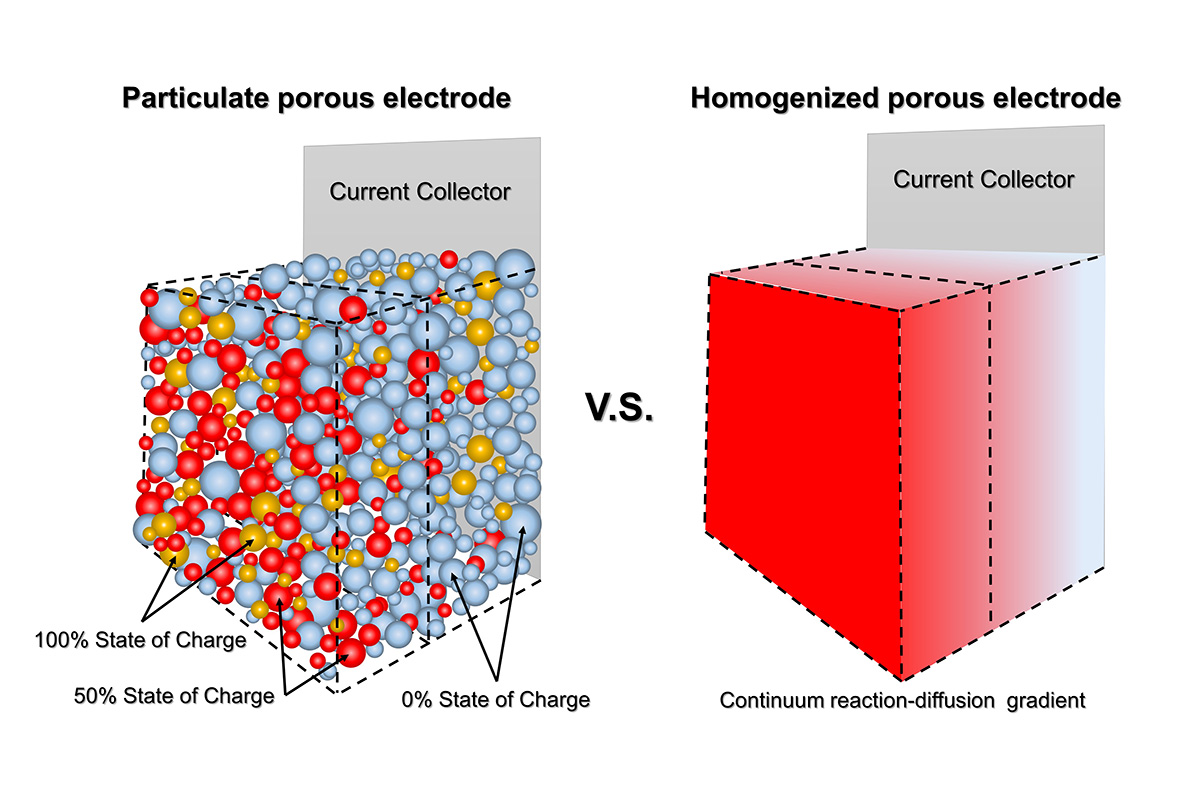
“We have been focusing on reaction heterogeneities in batteries because they are the birthplace of safety hazards,” Bai said. Here, the reaction heterogeneity refers to a local hot spot that has a much higher reaction current than the neighboring regions. The difference between the local current density over the hot spots and the apparent current density can become huge, potentially leading to everything from inefficiency to deadly accidents, depending on the nature of the electrode and the operating condition of the battery.
Part of the problem, Bai said, is the use of practical electrochemical models that in many cases assume a rather homogeneous reaction among the composing particles of the porous electrode. Better understanding how current is truly distributed through the real-world particulate porous electrodes is a task suited to the electrochemical engineer.
“This research is about the truth,” Bai said. “What is the true local current density? That is what we should pay attention to. And that is how we will gain confidence to design safer batteries.”
This NSF CAREER Award will help Bai’s lab further its understanding of the significance and evolution of spatiotemporal heterogeneities in batteries, complementing their earlier NSF project on the alkali metal anodes. Bai’s lab is specialized in designing mesoscale operando experiments using optical microscope. The combination of direct image analysis with physics-based mathematical models offers unique perspectives to understand fundamental electrochemistry in practical devices.
The project will benefit the education of graduate, undergraduate and K-12 students. A summer program is being established in collaboration with the Institute of School Partnership, which will invite underrepresented high school-age students and high school teachers to experience hands-on battery research and study the engineering concepts of equilibrium and dynamics.




