From mathematical concept to a clinical tool
Abhinav Jha to advance quantitative imaging with NIH grant
Clinicians often rely on imaging to make diagnostic and therapeutic decisions. The emerging paradigm of quantitative imaging promises to further strengthen the clinical role of imaging. A Washington University in St. Louis imaging scientist plans to develop a new framework to evaluate quantitative imaging methods using patient data without any gold standard.
Abhinav Jha, assistant professor of biomedical engineering in the McKelvey School of Engineering, will use a four-year, $1.83 million grant from the National Institutes of Health to develop this framework. He will then evaluate the efficacy on imaging data from patients with stage 3 non-small cell lung cancer, a disease with high prevalence and low survival rates.
Quantitative imaging (QI) is a relatively new technique that measures numerical or statistical features from images with the goal of using these features to make clinical decisions. Jha, who develops computational imaging methods for diagnosing and treating diseases, has been working on QI since 2007.
“QI is offering exciting new possibilities in improving diagnosis and therapies of diseases such as cancers and neurodegenerative disorders, as demonstrated in multiple research studies,” he said.
To translate this promise of QI, techniques to clinically evaluate QI methods based on how reliably they measure the underlying true quantitative value are needed. However, clinical evaluation requires availability of a gold standard which is time-consuming and expensive to obtain and is not always reliable.
“One of the challenges of evaluating quantitative imaging is determining if a quantitative feature has been measured reliably without knowing the truth,” he said.
To address this issue, Jha and his collaborators have developed no-gold-standard evaluation tools that build on previous findings by other researchers.
“We have a problem of huge importance, and we now have a tool, but the two haven’t gotten together in the clinic to solve the problem. This research proposes to take steps to bridge this translational gap.”
The grant will advance no-gold-standard evaluation technology to make it more clinically relevant and more practical to apply, which will all help with wider adoption and translation of this technology, Jha said.
Jha, who also has an appointment at Washington University’s Mallinckrodt Institute of Radiology, will collaborate with other faculty from the School of Medicine, including Barry Siegel, MD, professor of radiology and former chief of the Division of Nuclear Medicine; Joyce Mhlanga, MD, assistant professor of radiology; Richard Laforest, professor of radiology; and Clifford Robinson, MD, professor of radiation oncology. He also will collaborate with industry partners for wider dissemination of this tool.
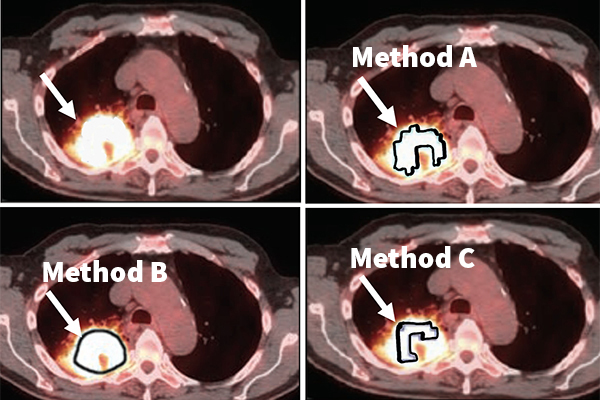
Jha, his collaborators and members of his Computational Medical Imaging lab will evaluate the efficacy of this no-gold-standard evaluation tool in evaluating different tumor segmentation methods on the task of measuring the volumetric features of metabolic tumor volume and total lesion glycolysis in images taken from patients with stage 3 non-small cell lung cancer.
While there are multiple treatment options for this disease, physicians do not have a sure way to determine which treatment is best for each patient. That’s where this tool comes in, Jha said.
“We will use this tool to identify the segmentation methods that yield the most reliable values for these volumetric features,” Jha said. “Our hypothesis is that the chosen segmentation method will provide a better prediction of whether a patient is responding or not early during the treatment. Accurate prediction of therapy response will help decide if the therapy must be continued or needs to be modified. Thus, this will help personalize therapy for the patient."
Specifically, Jha and his collaborators will measure these volumetric features from a type of PET scan used to detect metabolically active malignant lesions in patients with stage 3 non-small cell lung cancer. They also will look at the predictive ability of the metabolic tumor volume and total lesion glycolysis when combined with other data, such as the patient’s smoking history and the disease’s substage.
“Our purpose is to take this no-gold-standard evaluation tool to the next level and a step toward translation to the clinic,” he said.




