The quest for safer, more effective drugs for irregular heartbeat
Jianmin Cui will home in on the potassium ion channel with NIH grant
Irregular heartbeat, or arrhythmia, affects about 5 million people in the United States, and interestingly, some of the drugs used to treat the condition can also cause it. A biomedical engineer in the McKelvey School of Engineering at Washington University in St. Louis is going deep into the basic mechanisms that lead to arrhythmia to ultimately find potential new drug candidates.
Jianmin Cui, professor of biomedical engineering, will study the mechanisms of how voltage, a lipid known as PIP2 and a protein known as calmodulin (CaM) work together to activate the potassium ion channel in the heart that controls heart rhythm with a four-year, $2.5 million grant from the National Institutes of Health. By further understanding these ion channel mechanisms that control the electrical signaling in the heart, Cui and his team can look for target locations within the channel that may be a good site for new treatment options.
“This work is about how this channel is activated by voltage, CaM and PIP2,” Cui said. “We are trying to find the sites of the protein on this channel that are critical for the voltage sensor to activate and for the binding of PIP2 and CaM. We also want to study how the activation of the sensors occurs through the protein-protein interaction that opens the pore.”
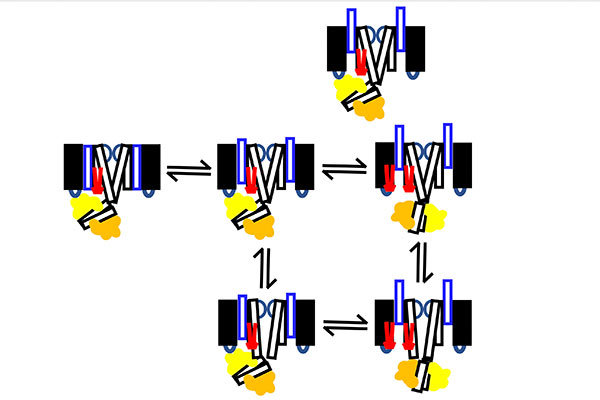
In the new research, Cui and his lab will investigate whether PIP2 binding to the voltage-sensing domain triggers the intermediate open to activated open transition. In addition, they will study whether the interaction between CaM and the voltage-sensing domain regulates the intermediate open to activated open transition. Finally, they will look to detect the change from a bent conformation to a straight conformation in the intermediate open to activated open transition.
“In the heart, the potassium ion channel only opens to the activated open state, because the association of KCNE1 suppresses the intermediate open state,” Cui said. “This slow transition of the ion channel to the activated open state is key to the role of the potassium ion channel in regulating heart rhythm. Our study will provide molecular mechanism for this important process.”
Once they understand this mechanism, they can look for sites on the channel protein on which they may be able to bind different small chemical compounds through a process known as molecular docking. By identifying the geometric and chemical traits of the small compounds, they can find the one that fits into the protein, similar to a jigsaw puzzle.
“The significance of this work is that we try to develop drugs that can modulate this channel to treat for arrhythmia,” Cui said. “We will target multiple sites and identify multiple lead compounds so we can choose the best, safest and most effective compounds.”




