Mapping the cell’s membrane-less compartments
WashU and St. Jude groups uncover the rules for organization of cellular condensates implicated in ALS
Cells are compartmentalized into distinct communities, with organelles and membranes keeping specific proteins and processes in one place. Interestingly, even without the benefit of a membrane, proteins and molecules can be concentrated into membraneless bodies known as biomolecular condensates. These condensates include bodies known as stress granules that form and dissolve in response to and the release of cellular stresses. Condensates come together in discrete areas, like a workplace breakout room. Aberrant formation and dissolution of condensates are implicated in a range of human diseases including Amyotrophic Lateral Sclerosis (ALS).
Since condensates are temporary structures, little is known about their organization. Do condensates have organization at all or are they haphazard messes of protein? A group led by Rohit Pappu, the Gene K. Beare Distinguished Professor of Biomedical Engineering in the McKelvey School of Engineering at Washington University in St. Louis, mapped out how condensates formed by prion-like domains (PLDs) of two proteins hnRNPA1 and FUS, are organized. Mutations in both the PLDs proteins are linked with increased risk of ALS. The research was published in Nature Communications Sept. 8.
“Are chemistries organized randomly in these blobs?” Pappu said. “Or is there specificity inside a condensate? Can you swim inside a condensate and see what is on the inside and what is on the interface?”
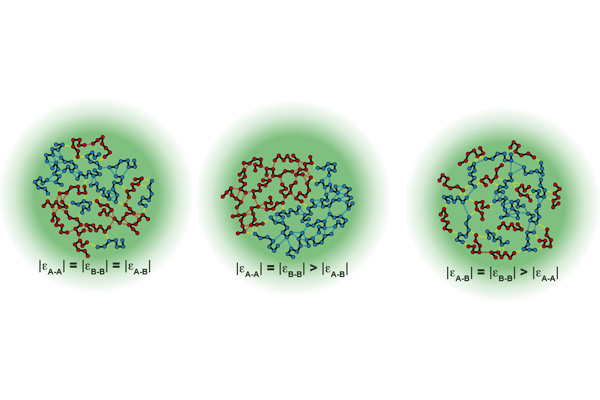
Most condensates are made up of multiple components and involve a network of homotypic (proteins of the same type) and heterotypic (proteins of a different type) interactions. Two worldviews have arisen from past research – that condensates are random mixtures of different protein domains, or that there are discrete territories within condensates that display some organization. This kind of organization is critical in disease development – condensates often form in times of cellular stress to sequester proteins, dissolving when the stress passes. If too many proteins of the same type interact, however, condensates can persist and disrupt and even kill cells. This is particularly a problem in cells which do not easily regenerate, such as neurons.
When the two proteins, hnRNPA1 and FUS, were present in equal amounts, heterotypic interactions dominated. But if the balance was tilted in favor of one of the proteins, homotypic interactions rapidly took over. Furthermore, proteins that engaged in strong interactions became buried towards the center of the condensate and were pushed away from the edges. Even protein length affected condensate behavior – longer proteins near the edges resulted in a thicker interface, where shorter proteins produced a thinner one.
Pappu said that this work will help define organizing principles for condensates that will define normal states and disease states and may even work for creating synthetic condensates down the line.
“A condensate is like a cellular battery or capacitor — we can ask how we can change the material properties to change the condensate’s output.”
Farag M, Borcherds WM, Bremer A, Mittag T, Pappu RV. Phase separation of protein mixtures is driven by the interplay of homotypic and heterotypic interactions. Nature, Sept. 8, 2023. https://doi.org/10.1038/s41467-023-41274-x
This work was supported by grants from the National Institutes of Health (R01NS121114), the St. Jude Research Collaborative on the Biology and Biophysics of RNP granules, and the Air Force Office of Scientific Research (FA9550-20-1-0241).




