May the force not be with you
Amit Pathak’s lab turns conventional wisdom about forces in cell migration on its head
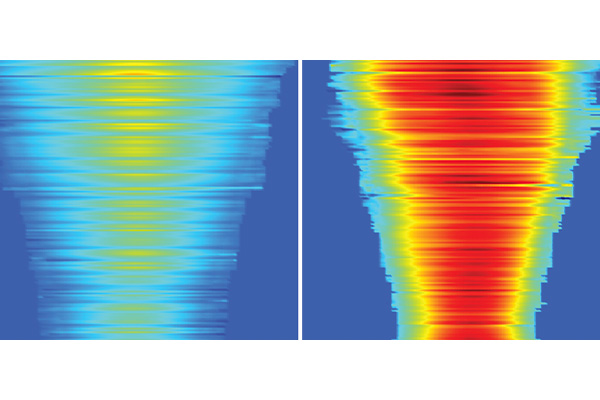
In mechanobiology, cells’ forces have been considered fundamental to their enhanced function, including fast migration. But a group of researchers in the McKelvey School of Engineering at Washington University in St. Louis has found that cells can generate and use lower force yet move faster than cells generating and using high forces, turning the age-old assumption of force on its head.
The laboratory of Amit Pathak, professor of mechanical engineering & materials science, found that groups of cells moved faster with lower force when adhered to soft surfaces with aligned collagen fibers. Cells have been thought to continually generate forces as they must overcome friction and drag of their environment to move. However, this conventional need for forces can be reduced in favorable environmental conditions, such as aligned fibers. Their results, published in PLOS Computational Biology Jan. 9, 2025, are the first to show this activity in collective cell migration.
Pathak and members of his lab have tracked the movement of human mammary epithelial cells for years, determining that cells move faster on a hard, stiff surface than on a soft surface, where they get stuck. Their research has implications in cancer metastasis and wound healing.
In the new research, they found that cells migrated more than 50% faster on aligned collagen fibers than on random fibers. In addition, they found that cells use aligned fibers as directional cues to guide their migration toward expanding their group.
“We wondered if you apply a force, and there's no friction, can the cells keep going fast without generating more force?” Pathak said. “We realized it's probably dependent on the environment. We thought they would be faster on aligned fibers, like railroad tracks, but what was surprising was that they were actually generating lower forces and still going faster.”
Amrit Bagchi, who earned a doctorate in mechanical engineering from McKelvey Engineering in 2022 in Pathak’s lab and is now a postdoctoral researcher at the Center for Engineering MechanoBiology at the University of Pennsylvania, went to great lengths to set up the research. Bagchi created a soft hydrogel in the laboratory of Marcus Foston, associate professor of energy, environmental & chemical engineering, over many months during the COVID-19 pandemic, then aligned the fibers using a special magnet at the School of Medicine before putting the cells on it to track their movement.
Bagchi developed a multi-layered motor-clutch model in which the force-generating mechanisms in the cells act as the motor, and the clutch provides the traction for the cells. Bagchi expertly converted the model for the collective cells using three layers — one for cells, one for the collagen fibers and one for the custom gel underneath — which all communicated with each other.
“Although the experimental results initially surprised us, they provided the impetus to develop a theoretical model to explain the physics behind this counterintuitive behavior,” Bagchi said. “Over time, we came to understand that cells use aligned fibers as a proxy for experiencing frictional forces in a way that differs significantly from the random fiber condition. Our model's concept of matrix mechanosensing and transmission also predicts other well-known collective migration behaviors, such as haptotaxis and durotaxis, offering a unified framework for scientists to explore and potentially extend to other interesting cell migration phenotypes.”
Bagchi A, Sarker B, Zhang J, Foston M, Pathak A. Fast yet force-effective mode of supracellular collective cell migration due to extracellular force transmission. PLOS Computational Biology, Jan. 9, 2025, DOI: https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1012664.
This work was supported by the National Institutes of Health (R35GM12876) and the National Science Foundation (CMMI:154857 and CMMI 2209684).
Codes used for generating simulations can be found at https://doi.org/10.5281/zenodo.13984567




