Imaging technology could better monitor tumor growth, drug effectiveness
More than 90% of drugs in development never make it to market after failing in the testing process, either in cells in the lab, in small animals or in humans. A biomedical engineer in the McKelvey School of Engineering at Washington University in St. Louis plans to improve on an existing imaging method that will give researchers more insight into the effects of drug candidates on tumor models over time.
To achieve this goal, Chao Zhou, associate professor of biomedical engineering, plans to use optical coherence tomography (OCT), a type of optical imaging technology that has been used for two decades in ophthalmology to take images of the retina. He will develop an improved version of the method to penetrate into 3D tumor models to determine the effect of drugs on their growth with a three-year, $855,305 grant from the National Institutes of Health's National Institute of Biomedical Imaging and Bioengineering.
Existing imaging technology measures drug efficacy on two-dimensional cell culture and can only see the surface of a tumor sample. However, tumors are three-dimensional. With existing technology, light cannot penetrate the depth of the tumor. In addition, researchers have to stain the sample to see it on a microscope, which destroys the sample.
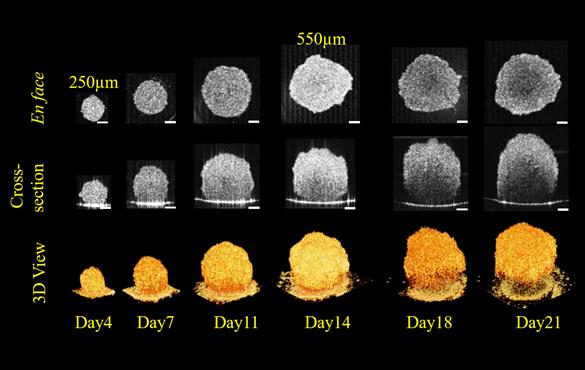
"With the OCT technology, we can image cells up to 1 to 2 millimeters deep," Zhou said. "That way we can see the surface as well as the center of the tumor and monitor it over time, say 3 to 4 weeks, to see how the tumor spheroid is growing and developing."
"This is important because the only other way to evaluate the internal structure of a tumor spheroid is to cut it open and see what's going on," Zhou said. "With this technology, we can follow the same tumor spheroids and see how they respond to different drug treatment."
Zhou's upgrade takes a chapter from parallel computing, in which many calculations can be performed at the same time, significantly speeding up the process. To enable parallel OCT imaging, he developed a technology called the space division multiplexing OCT in which they can use many beams of light simultaneously.
Zhou said conceptually, it is straightforward, but to implement it into hardware is challenging. He and his lab determined how to split the light and add optical delays between imaging channels to create different imaging signals.
"We can hear different stations on the radio because they are encoded in different frequencies so they don't overlap," he said. "What we are doing is basically the optical analogy of this kind of frequency modulation for OCT imaging. That way we can acquire all of the information from all different channels then put them together and create nice 3D pictures with high throughput."
Zhou and his lab use a stage that has 96 wells or up to 384 wells, each containing a 3D tumor spheroid sample. They expect to develop a parallel OCT imaging system that can generate 3D images from all of the wells in less than 20 minutes.
Zhou is collaborating with Marc Ferrer, leader, biomolecular screening and probe development at the National Institutes of Health on this R01 project.




