Wonderland
Focused ultrasound making strides in diagnosis, unlocking potential treatment of brain disorders

Our brains are designed for survival: They regulate metabolism, body temperature and myriad other functions that keep us alive. What if the brain could safely be put into a hibernation-like state to extend survival, for instance, for astronauts on an extended mission into space?
Such an idea has been bandied about since the 1960s, yet no safe and effective solution exists. Washington University in St. Louis researchers are the first to safely induce a hypothermic and hypometabolic state — the hibernation-like state — in small mammals by targeting the central nervous system using focused ultrasound.
We can push the limits of what’s possible, especially if we can reach deep into the brain without surgery. That opens up a new wonderland when we can interface with the brain without any incision.”
— Hong Chen
Hong Chen, associate professor of biomedical engineering in the McKelvey School of Engineering and of neurosurgery at WashU Medicine, is leading the way in focused ultrasound research, technology and clinical application in collaboration with colleagues in McKelvey Engineering and at WashU Medicine. Her growing research portfolio, most recently establishing the induction of the hibernation-like state, earned her a highly competitive National Institutes of Health Director’s Pioneer Award, which provides $5.4 million over five years to continue the work to develop a safe solution that may offer significant advancements in understanding metabolism regulation pathways and make extended space flights a reality.
Chen and her collaborators have used focused ultrasound to deliver drugs to the brain to treat brain diseases and to aid in noninvasive brain liquid biopsy for the molecular diagnosis of brain diseases.
There wasn’t a scientific term that fully captured the breadth of our work, so I coined the term ‘Neurosonics,’ which aims to turn discovery in neuroscience into health through ultrasound.”
— Hong Chen
“There wasn’t a scientific term that fully captured the breadth of our work, so I coined the term ‘Neurosonics,’ which aims to turn discovery in neuroscience into health through ultrasound,” she said. “Neurosonics integrates advances in neuroscience and ultrasonics to develop noninvasive and precise ultrasound technology to advance our understanding of brain function and transform the diagnosis and treatment of neurological disorders.”
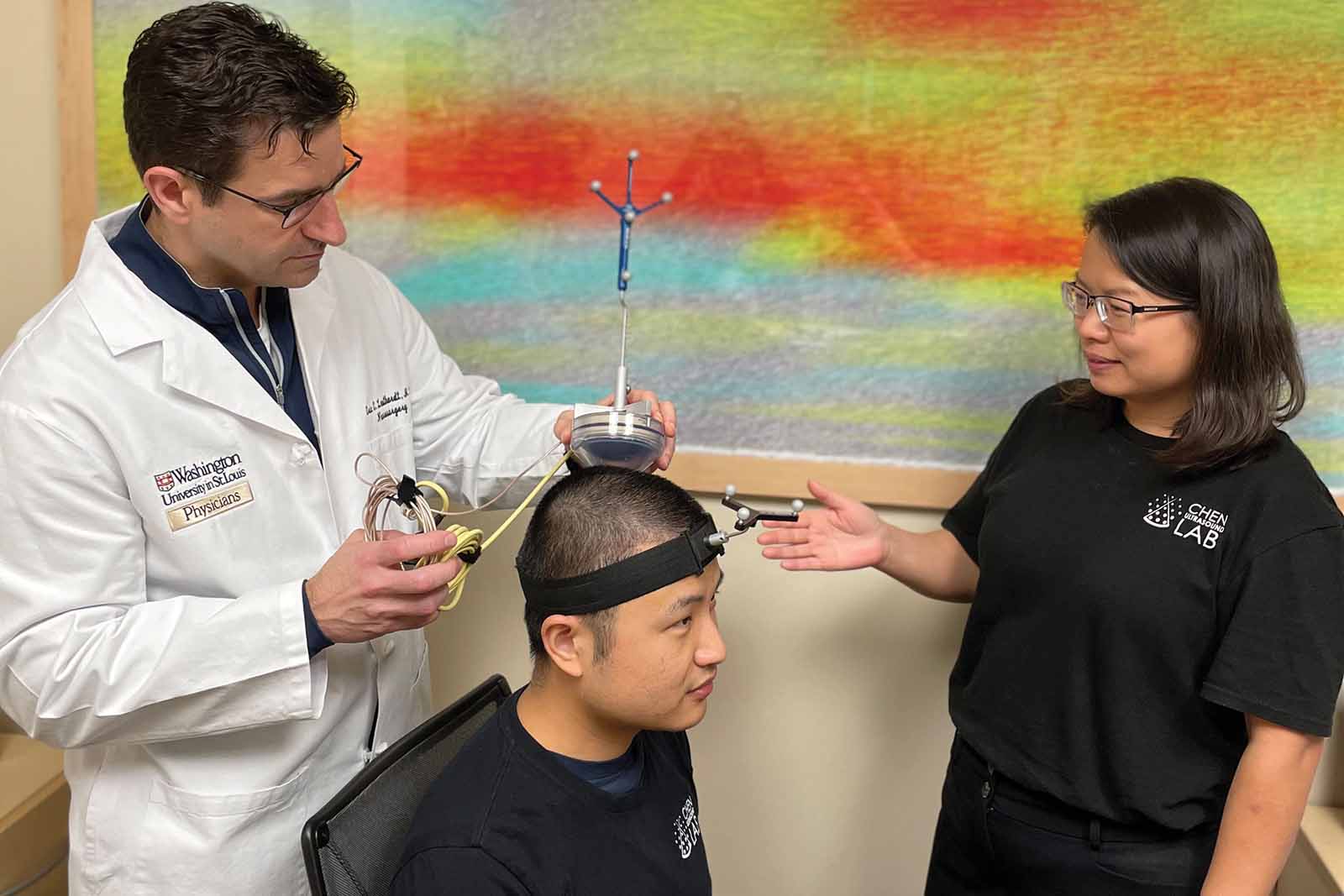
(From left) Eric Leuthardt, the Shi Hui Huang Professor of Neurological Surgery at WashU Medicine and of biomedical engineering and of mechanical engineering & materials science in McKelvey Engineering; graduate student Lu Xu; and Chen demonstrate the focused ultrasound device that has been granted U.S. Food and Drug Administration "Breakthrough Device" designation. (Credit: Hong Chen)
How it works
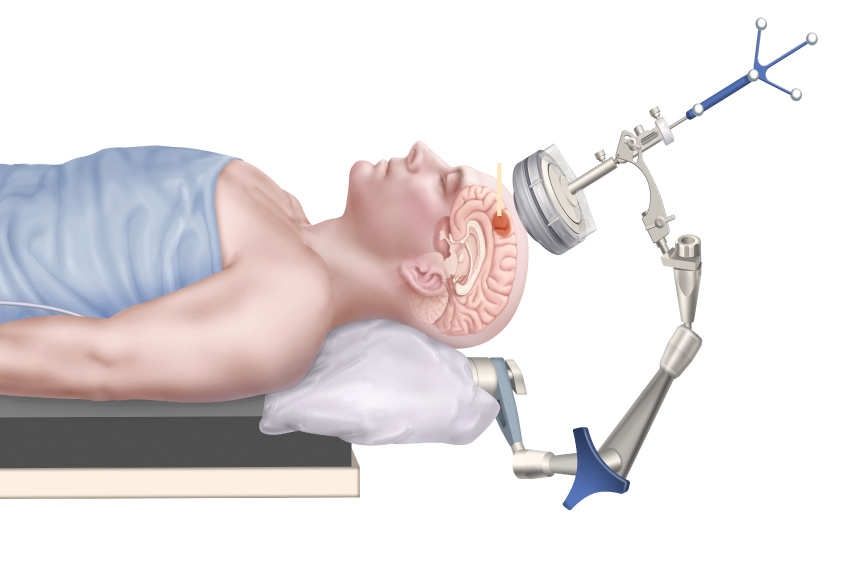
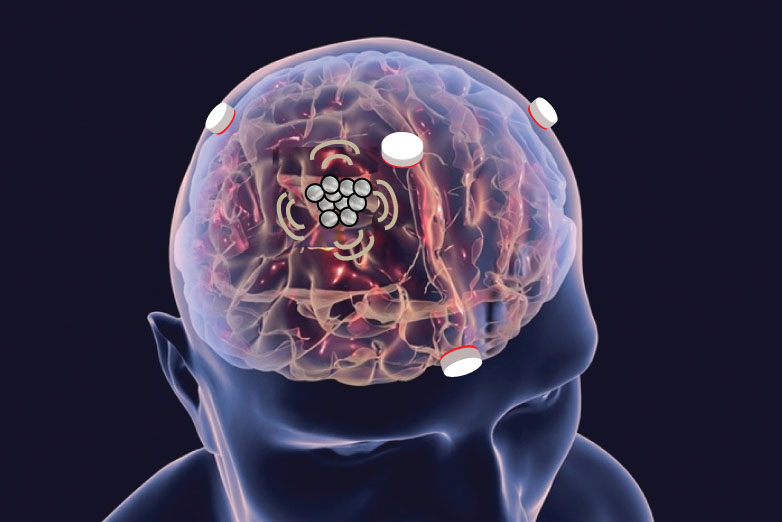
Focused ultrasound seems extremely complicated: It uses a special type of device to deliver ultrasound waves through the skull to precisely target a specific brain area, often deep in the brain. But the basic idea is as simple as using a magnifying glass to focus sunlight into a focal point. Focused ultrasound works in a similar way, but instead of sunlight, it uses sound waves. Ultrasound devices, which look like a shallow bowl or a helmet, send out multiple sound waves that are directed to meet at a specific point inside the brain. When these waves meet, they concentrate their energy in that tiny area. This focused energy can heat up or stimulate the brain tissue without needing to make an incision, which makes it a noninvasive way to interface with the brain.
“We can push the limits of what’s possible, especially if we can reach deep into the brain without surgery,” Chen said. “That opens up a new wonderland when we can interface with the brain without any incision.”
Among all forms of energy — such as light, electricity, magnetic fields and ionizing radiation — ultrasound is the only one that can safely and noninvasively penetrate the skull to target both shallow and deep brain regions in humans.
What it can do
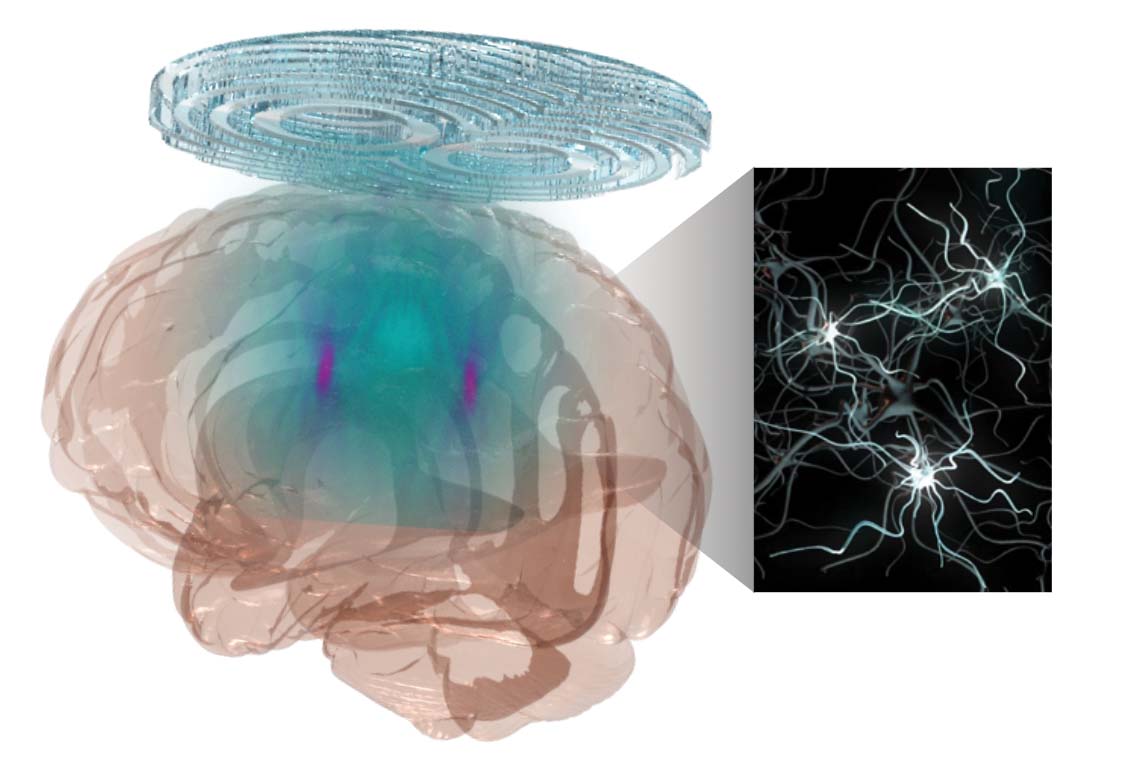
In the groundbreaking torpor research, published in May 2023 in Nature Metabolism, Chen and a multidisciplinary team induced a hibernation-like state in mice by using focused ultrasound to stimulate the hypothalamus preoptic area in the brain, which helps to regulate body temperature and metabolism. In addition to the mouse, which naturally goes into torpor, Chen and her team induced torpor in a rat, which does not.
For this work, she and her team, including Yaoheng (Mack) Yang, who earned a doctorate in biomedical engineering from McKelvey Engineering in 2022 and is now an assistant professor of biomedical engineering at the University of Southern California, created a wearable ultrasound transducer — the shallow bowl — to stimulate the neurons in the hypothalamus preoptic area. When stimulated, the mice showed a drop in body temperature of about 3 degrees C for about one hour. The mice’s metabolism showed a change from using both carbohydrates and fat for energy to only fat — a key feature of torpor — and their heart rates fell by about 47%, all while at room temperature.
In the rat, the team delivered ultrasound to the hypothalamus preoptic area and found a decrease in skin temperature, particularly in the brown adipose tissue region, as well as about a 1 degree C drop in core body temperature in their preliminary study.
Jonathan R. Brestoff, MD, PhD, associate professor of pathology & immunology at WashU Medicine, was a collaborator on the research. He and his lab worked with Chen’s team to take the measurements in the mice and provided insights, resources and experimental help to test the role of brown fat in focused ultrasound- induced torpor. Brestoff said the results have widespread implications and raise thoughtful questions.
“Can focused ultrasound technology be used to treat any diseases for patients or pets?” he asks. “If adapted for humans, could it enable long-term space flight? What are the implications of this technology for national defense and international affairs?”
Since joining WashU in 2015, Chen has established collaboration with more than 20 faculty members at WashU across 12 departments on projects funded by the NIH and other agencies.
Before the torpor research, Chen and her collaborators made other groundbreaking findings using focused ultrasound. Early in her tenure at WashU, Chen met Eric Leuthardt, MD, the Shi Hui Huang Professor of Neurological Surgery and professor of neuroscience at WashU Medicine and of biomedical engineering and of mechanical engineering & materials science in McKelvey Engineering, and the team has been innovating together ever since on what Leuthardt calls “an amazing progression.

When Chen joined WashU, Lihong Wang was assigned as her faculty mentor whom she met with regularly before he moved to CalTech.
Those meetings with Lihong were incredibly beneficial in the early phase of my career,” she said. “I still reach out to him for career advice. He is a true role model to me.”
— Hong Chen
“Hong and I have worked closely to develop sonobiopsy, which we have brought from a rodent demonstration to preclinical model to first in human trials, to now receiving an FDA Breakthrough Designation,” said Leuthardt, who also is vice chair of innovation in the Department of Neurosurgery, chief of the Division of Neurotechnology, and director of the Center for Innovation in Neuroscience and Technology and of the Brain Laser Center. “I love working with Hong because we make science fiction real. We have done that with touchless brain biopsies, and she has done that in several other domains, including ultrasound-induced hibernation. The implications for that are protean.”
Sonobiopsy uses focused ultrasound to target a precise location in the brain. Once located, the researchers inject microbubbles into the blood that travel to the ultrasound-targeted tissue and pulsate, which safely opens the blood-brain barrier. The temporary openings allow biomarkers, such as tau proteins, which are indicative of neurodegenerative disorders, to pass through the blood-brain barrier and release into the blood. The biomarkers can then be identified through a simple blood test. Conversely, the technique could also be used to facilitate drug delivery to the brain.
Chen and collaborators, including Leuthardt, published results in 2023 that were the first to open the door for noninvasive and targeted diagnosis and monitoring of neurodegenerative disorders with focused ultrasound- mediated liquid biopsy. In 2023, the team reported promising findings from the first-in-human prospective trial of sonobiopsy in patients with glioblastoma, a form of aggressive brain tumor that is commonly diagnosed through a risky and invasive surgical biopsy.
Chen credits the rapid progress of sonobiopsy to the collaborative research environment at WashU. “WashU is a place where the barriers between engineering and medicine have been removed,” she said. “Here, I can forge long-term collaborations with exceptional physician-scientists like Eric Leuthardt, which accelerates the clinical translation of our engineering innovations.”
Leuthardt said he believes sonobiopsy has the potential to fundamentally change how researchers interrogate the human brain, moving away from MRI and functional MRI.
“To understand the human brain on a molecular level currently requires me to insert a needle in the brain,” Leuthardt said. “With sonobiopsy, we now molecularly interrogate the brain. That will be a sea change in the questions we can answer about human brain function.”
Chen and her collaborators have been working on another focused ultrasound technology she calls sonogenetics, which integrates ultrasound with genetics to precisely modify neurons in the brain. In 2021, she published the first research that provided direct evidence showing noninvasive, cell-type-specific activation of neurons in the brain of a mammal by combining ultrasound- induced heating effect and genetics. It was also the first work to show that the ultrasound-genetics combination can control mouse behavior by stimulating a specific target deep in the brain. Their second-generation method, Sonogenetics 2.0, has the potential to combine the advantage of ultrasound and genetic engineering to modulate defined neurons noninvasively and precisely in the brains of humans and animals.

(From left) Jinyun Yuan, research scientist, and Chen look at a PCR machine in Chen's lab. Photo by Douglas Garfield
Looking ahead
Ever the planner, Chen develops five-year vision plans for her research. Now in Vision 3.0, she is focusing on expanding the field of Neurosonics through developing three research pillars that include sono-neuroengineering, sono-neuroscience and sono-neuromedicine.
Through Vision 3.0, Chen and her team’s mission is to innovate, impact and inspire. They are committed to innovating in Neurosonics to impact patient care and inspire new thinking in the scientific community.
“Our vision is to build on a lab culture that centers on three core values,” she said. “These include pioneering, integrity and teamwork.”
I love working with Hong because we make science fiction real.”
— Eric Leuthardt
While Chen and her collaborators are not the only researchers working on focused ultrasound, they are making remarkable waves in the field, which Leuthardt attributes to Chen.
“Hong is unstoppable in turning a vision into reality,” Leuthardt said. “She is tenacious in moving her creative ideas forward and validating them with vigor.”
Next steps
With the funding from the NIH Director’s Pioneer Award, Chen aims to test a bold hypothesis that the neural pathways governing the hibernation-like state are conserved across mammals, including humans.
“Throughout my career, I have continuously broken new ground in research and opened up new frontiers,” Chen said, “This award offers me, an ultrasound engineer, an exciting opportunity to venture into uncharted realms of neuroscience and pioneer innovative solutions in medicine.”