Brighter fluorescent markers allow for finer imaging of nanoscopic objects
Nanoparticles engineered by WashU researchers help provide a clearer picture of brain cell structure
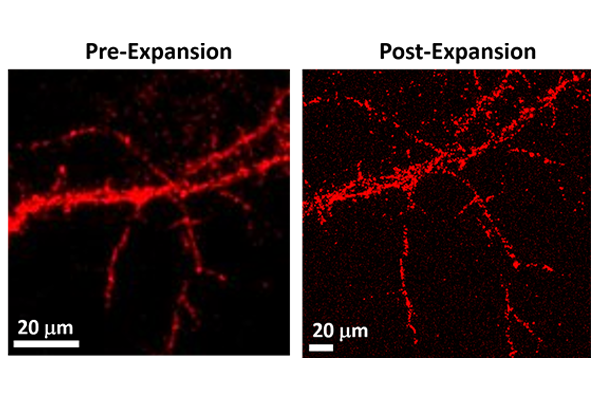
Researchers at the McKelvey School of Engineering at Washington University in St. Louis have pioneered a new technique that will enable higher-resolution imaging of very small objects like neurons. The technique, which improves on an existing method called expansion microscopy, is described in a new paper published in the journal Nano Letters June 12, 2023.
Most people are familiar with microscopes that use lenses to make an object appear larger and easier to see with the human eye. But expansion microscopy (ExM) works in practically the opposite way — by making the object itself larger. Scientists coat the sample — a cell, for example — with tiny light-emitting markers called fluorophores, then embed the sample within a gel that expands when it comes into contact with water. As the sample grows larger, the fluorescent labels trace the outlines of features too small to see, like the thin branches, or dendrites, that grow from brain cells.
But expansion microscopy has a major shortcoming. The light signal emitted by conventional fluorophores loses much of its intensity (more than 50%) during the preparation and expansion steps. “When you make things bigger, it’s not necessarily good, because if you don't change the amount of signal that is already present, that signal gets weaker,” said Barani Raman, professor of biomedical engineering.
Raman and Srikanth Singamaneni, the Lilyan & E. Lisle Hughes Professor in the Department of Mechanical Engineering & Materials Science, have addressed this issue by using ultrabright fluorescent markers called plasmonic-fluors (PFs). Singamaneni developed the PFs for other applications in 2020.
“It's a nice example of two people with completely different expertise having a chance conversation and saying ‘Okay, this problem is here in one field, but the solution is there in another field,’” Raman said. The team has dubbed its new technique “plasmon-enhanced expansion microscopy,” or p-ExM.
The plasmonic-fluor is constructed from a core particle of gold wrapped in a silver shell, which is then covered with a layer of other materials, including conventional fluorophores. The structure is designed to protect the fluorophores from the harsh chemicals used in the process and to make the light signal from the fluorophores much brighter. The technique will aid researchers in mapping neural networks, or the connections between neurons.
“The metal nanoparticle serves as an antenna, which means that it is able to pull more light into the fluorophores,” Singamaneni said. The interaction between the gold-silver nanoparticle and the fluorophores also causes the fluorophores to emit more photons than they normally would. As a result, the plasmonic-fluor is nearly four orders of magnitude brighter than the fluorescent markers would be on their own. Plasmonic fluors also solve the problem of signal dilution because the fluorescent markers are attached directly to the nanoparticle, so they don’t spread apart when the sample expands.
To demonstrate the potential of plasmon-enhanced expansion microscopy, the researchers used it to study a sample of neurons from the hippocampus region of the brain. In some cases, the neuron’s budding branches, called neurites, are too close to each other to make out without the help of techniques like expansion microscopy.
“When two neurites are too close to each other, we cannot resolve them. The software thinks that they are just one neurite,” Singamaneni said.
After labeling the cells with the ultrabright plasmonic-fluors and expanding the sample, the team was able to count the number of neurites, quantify the total area of the neurites and measure the length of individual neurites. They identified 2.5 times more neurite terminal points than were visible before the sample was expanded.
When the team compared the plasmonic-fluor’s performance to the fluorophores by themselves, they found that the plasmonic-fluor retained about 76% of the light signal while the fluorophores retained less than 16%. The team’s results also showed that plasmon-enhanced expansion microscopy is compatible with existing expansion microscopy protocols, which means plasmonic fluors can be used in place of conventional fluorophores in future studies. PFs can also be created from any given fluorophore that suits researchers’ needs.
Rathi P, Gupta P, Debnath A, Baldi H, Wang Y, Gupta R, Raman B, Singamaneni S. Plasmon-Enhanced Expansion Microscopy. Nano Letters June 12, 2023. DOI: https://doi.org/10.1021/acs.nanolett.3c01256
This research was supported by the Air Force Office of Scientific Research and the National Science Foundation.





